Impactor Quality Control — General Principles
Applied Particle Principles, LLC, first articulated the concept of “effective diameter” and the principles of multi-nozzle impactor quality control in 2005. These principles have gradually become standard industry practice. And in 2019, Pharm. Forum proposed formal adoption of these approaches into the next revision of USP Section 601.
Optical Inspection of Impactor Nozzles
Optical inspection of nozzles is the standard method for maintaining cascade impactors. Read about how to identify high-quality nozzles, including nozzle roundness and debris upstream of the nozzle exit (Roberts and Lavarreda, 2011).

Impactor Quality Control via Stage Pressure Drop
Stage pressure drop — a valid surrogate for optical inspection — FAST, INEXPENSIVE, NO SHIPMENT OF IMPACTORS TO INSPECTION VENDOR
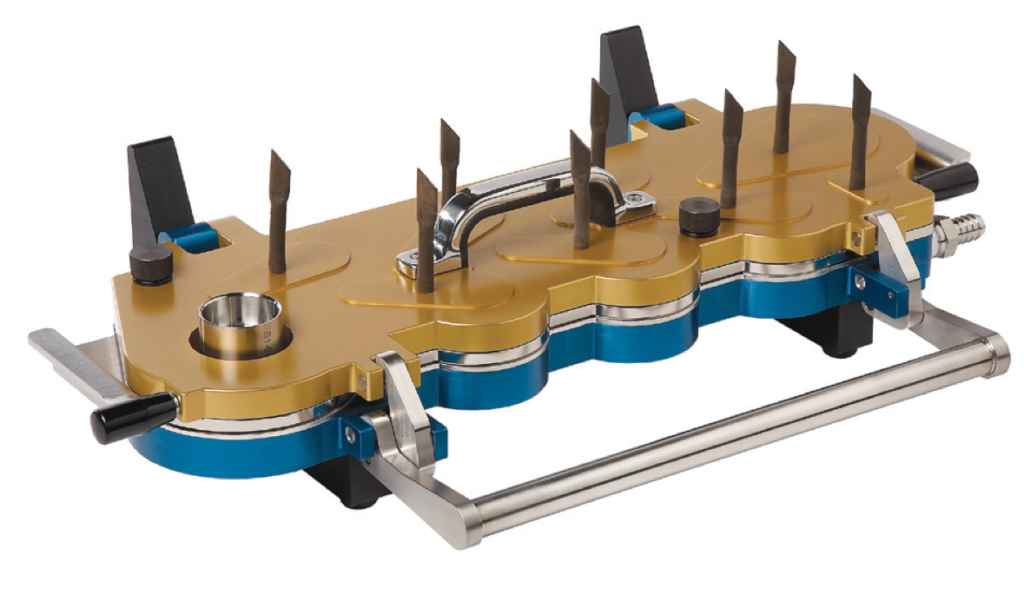

Impactor Quality Control for Registered Drug Products
Once the FDA-approved product specifications are known, the quality of the impactors performing batch release testing must ensure that the product specifications can be quantitatively known to within a precision dictated by the product specifications themselves. Often, these impactor quality requirements are more lax than those necessary for proper investigation of new products, such as the testing of early-stage new formulations or for preparation of batches for clinical trials. Prudent consideration of these issues enables cost-saving measures in many cases at the same time as reduced risk of out-of-specification impactor problems.
Latest publication in AAPS Pharm. Sci. Tech. (https://rdcu.be/bfRLM)